Did you know that L-dopa was first discovered by Markus Guggenheim in 1913, and that he isolated it from the Vicia faba plant?
Furthermore, did you know that numerous scientific studies from expert teams worldwide have demonstrated that the Vicia faba plant is effective in combating nearly all Parkinson’s symptoms? These studies also highlight the significant neuroprotective potential of Vicia faba in slowing the progression of the disease. You can find some of these studies at the end of this article.
Our journey with AtremoPlus, a Vicia faba-based nutraceutical, began in 2009 when a team of highly experienced experts in neurodegenerative diseases set out to develop a solution that offers rapid symptomatic relief for Parkinson’s disease AND also helps to slow disease progression.
The goal was clear: the solution had to be natural and free from side effects, yet standardized in L-Dopa to ensure safe and consistent dosing. It also needed to include Carbidopa to facilitate the crossing of L-Dopa through the blood-brain barrier. Additionally, the nutraceutical required potent neuroprotective components to address a long-standing need in the Parkinson’s community: a disease-modifying action that contributes to slowing the progression of the disease.
The Vicia faba plant emerged as the perfect candidate to meet these objectives.
The Conception Phase 2009-2011
With over 120 varieties of Vicia faba, the first challenge was identifying the variety with the highest L-Dopa content. Research revealed that it is not the beans but the structural parts of the plant that contain the highest levels of L-Dopa.
Once the best variety was found, the focus shifted to optimizing the cultivation process and developing a gentle extraction method to preserve the active compounds. This includes not only L-Dopa and Carbidopa but also numerous vitamins, minerals, amino acids, flavonoids, carotenoids, and polyphenols, all crucial for achieving our overall goals.
After achieving this target, the final step in the development phase was the standardization of the product’s L-Dopa content, which was achieved with a normalization of 21.5 mg/g. A standardized solution was an essential target for ensuring regular and safe dosage.
The Preclinical and Clinical Testing Phase from 2011- ongoing until now
Even though it is not a regulatory requirement to test food supplements preclinically and clinically, we recognized the importance of these steps in developing AtremoPlus. This ensures we offer a safe and effective solution to the Parkinson’s disease community and helps us understand the disease-modifying mechanisms at work in AtremoPlus.
Numerous preclinical studies have been performed.
The essential preclinical and clinical findings discovered so far showed that AtremoPlus:
- is a natural source of L-Dopa and Carbidopa that crosses the blood-brain barrier.
- shows an excellent safety profile as it is well tolerated with no side effects for 100% of study participants.
- is standardized to 21.5 mg/g of L-dopa for safe and consistent dosing and allowed during trials a reduction of the antiparkinson medication by 25-50%.
- has shown exceptional neuroprotective results in preclinical trials reducing oxidative damage and subsequent chronic inflammatory processes, both recognized as main triggers in the pathogenesis of PD.
- significantly increased norepinephrine, an essential component in motor coordination, cognitive performance, mood, and energy dynamics.
- improved overall methylation, a biomarker involved in proper protein defolding in the brain (epigenetic improvement) to reduce plaques aggregated through protein misfolding.
- improved UPDRS (Unified Parkinson’s Disease Rating Scale) test scores by 50% in the Parkinson’s group without cognitive impairment, 46% in the group with mild cognitive impairment, and 20% in the group with Parkinson’s and dementia.
- accelerated brainwaves from very slow waves (Delta and Theta, associated with daytime sleepiness and chronic fatigue) to Alpha waves (relaxed but awake).
- reduced stress biomarkers such as cortisol and prolactin.
Note: Most users take AtremoPlus alongside conventional antiparkinson’s medications for enhanced quality of life.
Example brain waves improvement:
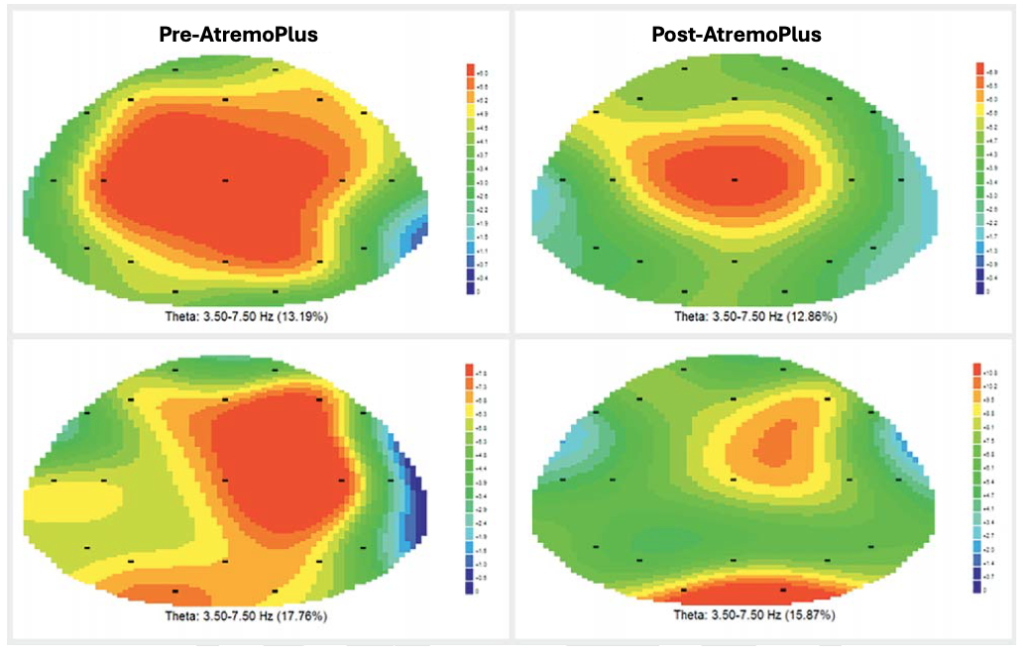
Image: Before and after 5g intake of AtremoPlus and after 1h. The red areas correspond to slow Theta waves, often associated with drowsiness and day sleepiness, while Alpha waves, shown in green, are faster. They indicate a state of relaxation while remaining awake and alert.
The long-term experience since the market launch in 2016 and until now
Over the last eight years, we have gathered valuable insights into the long-term user experience with AtremoPlus.
Users and their neurologists report the following:
- They are surprised and happy not only with the fast results in most motor, cognitive, and mood issues but also with the long-term stability, extending more than eight years for the earliest users.
- AtremoPlus is generally not subject to “wearing off,” as often observed with synthetic L-Dopa.
- “Off-moments” and “freezing” are reduced, and transitions between “on” and “off-moments” are smoother.
- AtremoPlus often has highly beneficial effects for people considered at the end of their therapeutic journey, even after years and decades of dopaminergic deficiency, strong wearing off, and freezing.
- Improved sleep quality with better regeneration.
- Less pain.
- The ability to resume activities that were previously abandoned.
- Better mood and increased confidence in their capabilities.
- Improved fine motor skills.
- More strength in legs and arms.
- Reduced tremors.
- Less rigidity and better mobility.
- Better concentration and focus.
- Less day sleepiness and chronic fatigue.
- Better memory.
- Increased motivation.
Overall, users report a better general sense of well-being.
You can find our testimonial page by clicking here: Atremoplus testimonials.
Who is AtremoPlus suitable for?
Due to its enhanced properties, AtremoPlus is suitable for individuals with Parkinson’s disease at all stages.
Recent studies with our food supplement have demonstrated excellent results with individuals suffering from Alzheimer’s disease and dementia.
However, AtremoPlus is particularly indicated for three specific groups:
1. Individuals who have reached the late stages of their therapeutic journey. AtremoPlus often delivers outstanding results for those who have lived with Parkinson’s disease for many years or even decades, experiencing significant ‘wearing off’ effects.
2. Individuals who experience severe side effects from synthetic L-Dopa and require finding alternative solutions.
3. Individuals looking for a plant-based vegan and natural solution that includes protective properties against oxidative stress and chronic inflammation.
Outlook for the Parkinson’s community
AtremoPlus offers comprehensive nutritional value and active ingredients. Alongside L-Dopa and Carbidopa, it features a unique combination of nearly all vitamins, minerals, amino acids, carotenoids, flavonoids, and polyphenols, recognized for their extensive health benefits.
Some of these active ingredients are even known to enhance neurorestorative compounds such as BDNF (brain-derived neurotrophic factor), which plays a crucial role in neuroscience and brain plasticity by promoting neurogenesis and synaptogenesis.
Our experience shows that AtremoPlus can significantly improve the quality of life for individuals suffering from Parkinson’s disease at all stages. We hope it becomes better known within the Parkinson’s community, which often seeks solutions desperately.
AtremoPlus is produced Eruope in a facility that is certified GMP (good manufacturing practices) and ISO 9001.
We can provide a datasheet on optimized usage and a research summary. Due to regulatory reasons, these materials can only be provided to healthcare professionals. For inquiries, please contact us at: contact@atremoplus.com
This content may be important for people who need this natural solution. Thanks for sharing !
By clicking on the button below, I leave the information site:
Disclaimer:
Please note that this blog provides information about our dietary supplement AtremoPlus and related topics.
AtremoPlus is not intended to treat, prevent or cure any disease, including Parkinson’s.
This blog is not intended to provide medical advice.
If you have any medical questions, please contact your healthcare professional.
Some References :
Voici la liste triée par ordre alphabétique des auteurs et sans doublons :
1. Abdel-Sattar, Essam, et al. “Methanolic extracts of a selected Egyptian Vicia faba cultivar mitigate the oxidative/inflammatory burden and afford neuroprotection in a mouse model of Parkinson’s disease.” Inflammopharmacology 29 (2021): 221-235.
2. Apaydin, Hülya, Sibel Ertan, and Sibel Özekmekçi. “Broad bean (Vicia faba)—A natural source of L‐dopa—Prolongs “on” periods in patients with Parkinson’s disease who have “on–off” fluctuations.” Movement disorders: official journal of the Movement Disorder Society 15.1 (2000): 164-166.
3. da Costa, Ianara Mendonça, et al. “Supplementation with herbal extracts to promote behavioral and neuroprotective effects in experimental models of Parkinson’s disease: a systematic review.” Phytotherapy research 31.7 (2017): 959-970.
4. Dhull, Sanju Bala, et al. “A review of nutritional profile and processing of faba bean (Vicia faba L.).” Legume Science 4.3 (2022): e129.
5. Espay, Alberto J., Peter A. LeWitt, and Horacio Kaufmann. “Norepinephrine deficiency in Parkinson’s disease: the case for noradrenergic enhancement.” Movement Disorders 29.14 (2014): 1710-1719.
6. Fuentes-Herrera, Paula Beatriz, et al. “Content and Yield of L-DOPA and Bioactive Compounds of Broad Bean Plants: Antioxidant and Anti-Inflammatory Activity In Vitro.” Plants 12.23 (2023): 3918.
7. Gravesteijn, Elske, Ronald P. Mensink, and Jogchum Plat. “Effects of nutritional interventions on BDNF concentrations in humans: a systematic review.” Nutritional neuroscience 25.7 (2022): 1425-1436.
8. He, Xuetao, et al. “The patterns of EEG changes in early-onset Parkinson’s disease patients.” International Journal of Neuroscience 127.11 (2017): 1028-1035.
9. Kempster, P. A., and M. L. Wahlqvist. “Dietary factors in the management of Parkinson’s disease.” Nutrition reviews 52.2 (1994): 51.
10. Latif, Saad, et al. “Dopamine in Parkinson’s disease.” Clinica chimica acta 522 (2021): 114-126.
11. Morais, L. C. S. L., J. M. Barbosa-Filho, and R. N. Almeida. “Plants and bioactive compounds for the treatment of Parkinson’s disease.” Arquivos Brasileiros de Fitomedicina Científica 1 (2003): 127-132.
12. Nikkhah, Karim, et al. “Efficacy and safety of Vicia faba L. extract compared with levodopa in management of Parkinson’s disease and an in-silico phytomedicine analysis.” International Journal of Ayurvedic Medicine 14.3 (2023): 794-800.
13. Orlando, Giustino, et al. “Inhibitory effects induced by Vicia faba, Uncaria rhyncophylla, and Glycyrrhiza glabra water extracts on oxidative stress biomarkers and dopamine turnover in HypoE22 cells and isolated rat striatum challenged with 6-hydroxydopamine.” Antioxidants 8.12 (2019): 602.
14. Qi, Guoyuan, et al. “Neuroprotective action of tea polyphenols on oxidative stress-induced apoptosis through the activation of the TrkB/CREB/BDNF pathway and Keap1/Nrf2 signaling pathway in SH-SY5Y cells and mice brain.” Food & function 8.12 (2017): 4421-4432.
15. Rabey, J. M., et al. “Improvement of parkinsonian features correlate with high plasma levodopa values after broad bean (Vicia faba) consumption.” Journal of Neurology, Neurosurgery & Psychiatry 55.8 (1992): 725-727.
16. Rijntjes, Michel. “Knowing your beans in Parkinson’s disease: a critical assessment of current knowledge about different beans and their compounds in the treatment of Parkinson’s disease and in animal models.” Parkinson’s Disease 2019 (2019): 1349509.
17. Rijntjes, Michel. “Effects of Parkinson’s disease on brain-wave phase synchronisation and cross-modulation.” Europhysics Letters 89.4 (2010): 48001.
18. Ryu, Jaihyunk, et al. “Fatty acid composition, isoflavone and L-3, 4-dihydroxyphenylalanine (L-dopa) contents in different parts of faba bean (Vicia faba) genotypes.” Plant breeding and Biotechnology 5.4 (2017): 314-324.
19. Shetty, Kalidas, Reena Randhir, and Dipayan Sarkar. “Bioprocessing strategies to enhance L-DOPA and phenolic bioactives in the fava bean (Vicia faba).” Functional Foods and Biotechnology. CRC Press, 2019. 99-114.
20. Shinde, Sagar, Athoiba Singh, and Shekhar Shinde. “Emerging Perspectives on Plant-Based L-Dopa Sources for the Treatment of Parkinson’s disease: A Review.” Bull. Env. Pharmacol. Life Sci 12 (2023): 11-17.
21. Shirahige, Livia, et al. “Quantitative electroencephalography characteristics for Parkinson’s disease: a systematic review.” Journal of Parkinson’s Disease 10.2 (2020): 455-470.
22. Sathya Prabhu, D., and V. Devi Rajeswari. “Nutritional and biological properties of Vicia faba L.: A perspective review.” International Food Research Journal 25.4 (2018): 1332-1340.
23. Stumpf, Kilian, et al. “Effects of Parkinson’s disease on brain-wave phase synchronisation and cross-modulation.” Europhysics Letters 89.4 (2010): 48001.
24. Topal, Nurdoğan, and Hatice Bozoğlu. “Determination of L-Dopa L-3, 4-dihydroxyphenylalanine Content of Some Faba Bean Vicia faba L. Genotypes.” Journal of Agricultural Sciences 22.2 (2016): 145-151.
25. Vered, Y., et al. “Bioavailability of levodopa after consumption of Vicia faba seedlings by Parkinsonian patients and control subjects.” Clinical neuropharmacology 17.2 (1994): 138-146.
